Severe pulmonary aspergillosis post viral illness in immunocompetent patients: A case series
Abstract
Invasive pulmonary aspergillosis (IPA) is a life-threatening disease commonly affecting the lungs of immunosuppressed patients. Recently, Aspergillosis was diagnosed in immunocompetent patients with viral pneumonia due to influenza virus and coronavirus-19 infections. A series of four immunocompetent patients from different hospitals in Lebanon diagnosed with pulmonary aspergillosis post viral infection is reported. Aspergillosis is an important differential diagnosis in patients with viral infections and worsening infiltrates on chest x-ray, in addition to bacterial and mycobacterial infections with which they share common features. Fungal infections should be suspected early, and treatment should be considered knowing that these infections entail worse outcomes.
Introduction
Aspergillosis is due to infection with one of the Aspergillus species and is associated with high morbidity and mortality in patients with immunodeficiencies; some examples include neutropenia, post-transplant, acquired immunodeficiency syndrome, chronic structural lungs disease or chronic corticosteroids use. The lung is the most commonly affected organ. The term chronic pulmonary aspergillosis is used to describe the disease in immunocompetent patients with a more chronic and less invasive infection whereas invasive pulmonary aspergillosis (IPA) defines acute aggressive infection that occurs most commonly in the immunosuppressed patients and leads to respiratory failure and death. But recently, IPA was diagnosed in immunocompetent patients with viral pneumonia due to influenza virus and Coronavirus-19 (COVID-19) infections. Our cases include four immunocompetent patients that presented with acute respiratory symptoms due to aspergillus infection following different viral infections.
Cases
Case 1
A 52-year-old female patient with history of asthma, hypertension, dyslipidemia and fibromyalgia presented for severe dyspnea and respiratory failure. On physical examination: blood pressure was 150/80 mmHg, heart rate 90 beats/min, temperature 37.5 °C and arterial oxygen saturation 94% on 10L/min of oxygen by facemask. She had bilateral wheezing and tachypnea. Her blood tests upon admission showed leucopenia (WBC = 2.39 × 109 per L), thrombocytopenia (platelets = 136 × 109 per L) and elevated CRP (28.8 mg/dL). Her electrolytes, creatinine and liver function tests were within normal limits. HIV serology and autoimmune workup (including P-ANCA, C-ANCA, ANA, rheumatoid factor and anti-CCP antibodies) were done due to recurrent asthma exacerbation and were negative. She was diagnosed with influenza pneumonia with superimposed bacterial infection and asthma exacerbation and was started on Oseltamivir, Levofloxacin, Albuterol/Ipravent and intravenous methylpredisolone. Her stay was complicated by severe dyspnea with respiratory failure requiring transfer to the intensive care unit (ICU).
CT chest showed bilateral lung consolidations in the posterior segments of the lower lobes associated with nodular and tree in bud infiltrates. Aspergillosis was suspected and bronchoscopy was done showing ulceration of the trachea with pseudomembrane and diffuse inflammation (Fig. 1 , Fig. 2). Biopsy was taken showing branching septated hyphae compatible with Aspergillus hyphae (Fig. 3). The patient was diagnosed as aspergillus tracheobronchitis. She was treated empirically with Voriconazole, and the treatment was continued after diagnosis confirmation by biopsy. However due to limited improvement, Anidulafungin was added to Voriconazole to continue double fungal coverage for 3 weeks. The patient improved with resolution of symptoms and thrombocytopenia and normalization of inflammatory markers and was discharged on oral Voriconazole for 6 additional months.
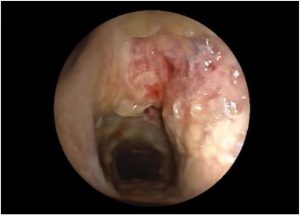
Fig. 1. Bronchoscopy showing airways ulceration.
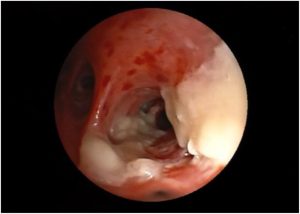
Fig. 2. Bronchoscopy showing pseudomembranes.

Fig. 3. Pathology slide showing branching septated hyphae.
Case 2
A 41-year-old male patient presented to the emergency department for dyspnea of 5 days duration associated with fever, productive cough, desaturation and diarrhea. No past medical or surgical history was reported. Patient was a heavy smoker (>80 pack-year). On physical examination: blood pressure was 140/70 mmHg, heart rate 124 beats/min, temperature 36.7 °C and arterial oxygen saturation 75% on 10L/min of oxygen by facemask. The patient was in severe respiratory distress with bilateral decrease in breath sounds and bilateral wheezing. Abdominal breathing was noted. Intubation was done urgently for mixed hypercapnic and hypoxic respiratory failure and the patient was admitted to the ICU.
His blood tests upon admission showed leukocytosis (WBC = 27 × 109 per L) with elevated procalcitonin (0.665 ng/ml) and CRP (27.3 mg/dL). Other labs including HIV serology were normal. Chest x-ray showed bilateral lung infiltrates compatible with viral pneumonia. Chest CT scan showed diffuse bilateral patchy and tree in bud infiltrates(Fig. 4). Urgent bronchoscopy was done and bronchoalveolar lavage (BAL) cultures were taken. Patient was started on Oseltamivir and broad-spectrum antibiotics (Levofloxacin, Ceftriaxone and Vancomycin) for coverage of superimposed bacterial infection on top of suspected influenza pneumonia (influenza polymerase chain reaction (PCR) was not done). Intravenous methylprednisolone was needed in the setting of severe COPD exacerbation with bronchospasm.
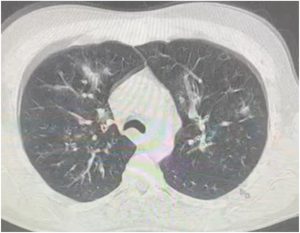
Fig. 4. CT chest showing multiple ground glass opacities with tree in bud infiltrates.
BAL and sputum culture showed Aspergillus infection and Voriconazole was started. Patient developed severe septic shock refractory to increasing norepinephrine dose. Severe mixed respiratory and metabolic acidosis refractory to bicarbonates administration and increasing minute ventilation was noted on arterial blood gases. Chest x-ray showed bilateral opacities with normal cardiac ultrasound and increasing oxygen requirement compatible with acute respiratory distress syndrome (ARDS). Patient passed away due to persistent hypoxia despite maximal ventilatory support.
Case 3
A 67-year-old male patient previously healthy presented to the clinic for recurrent dyspnea on minimal exertion, desaturation and productive cough 1 week post severe COVID-19 pneumonia being treated with oral prednisone for fibrotic lung changes post COVID-19 pneumonia. On physical examination: blood pressure was 120/70 mmHg, heart rate 75 beats/min, temperature 36.2 °C and arterial oxygen saturation 87% on room air. CT chest showed a tree in bud opacity with a 22 mm cavitary mass like consolidation in the right upper lobe suspicious of tuberculosis or fungal infection. Tuberculin skin test and acid-fast bacilli smear were negative. Bronchoscopy was done in outpatient setting and BAL showed Aspergillus infection which was treated successfully with Voriconazole. CT chest repeated after 3 and 5 months confirmed clearance of the infection with shrinkage of the cavitary mass. Bronchoscopy was repeated after the second CT chest and confirmed clearance of Aspergillus infection.
Case 4
An 80-year-old male patient with history of heart failure with preserved ejection fraction and limited activity (ambulate with assistance) presented to the clinic with severe dyspnea and desaturation 3 weeks post COVID-19 pneumonia. On physical examination: blood pressure was 130/70 mmHg, heart rate 88 beats/min, temperature 37.3 °C and arterial oxygen saturation 86% on room air. CT chest showed large cavity in the right upper lobe compatible with Aspergilloma(Fig. 5). BAL showed Aspergillus infection and positive galactomannan level. He was treated successfully with voriconazole and was discharged home but was lost to follow up.

Fig. 5. CT chest showing a large cavity in the right upper lobe.
Discussion
Pulmonary aspergillosis can cause multiple diseases that includes hypersensitivity reaction (allergic broncho-pulmonary aspergillosis, hypersensitivity pneumonitis), Aspergilloma and chronic pulmonary aspergillosis in damaged lung and IPA.
Severe aspergillosis infections were identified recently in patients who have viral pneumonia even if they are not immunosuppressed. Here we present four cases with different viral infections who were identified to have different forms of Aspergillus infection.
The common lesions on CT scan suggestive of aspergillosis are related to either airway invasion or angioinvasion. Airway invasion includes diffuse centrilobular nodules with branching linear and nodular areas (tree in bud), multifocal areas of nodular consolidation and surrounding ground glass opacities, and tracheal and bronchial wall thickening; whereas angioinvasion includes patchy consolidation with surrounding ground glass opacity within the first 14 days of the disease (Halo sign) and nodular lesion with central hypodensity (hypodense sign) that breakdown with air crescent formation within the consolidation after 14 days of the disease (air crescent sign). All our patients had suspicious CT chest findings (tree in bud opacities or cavitations) that triggered IPA suspicion.
Diagnosis can be confirmed by finding Aspergillus on biopsy or culture from BAL [8]. A recent criteria for IPA diagnosis in ICU patients requires having IPA signs and symptoms, abnormal lung imaging findings and positive Aspergillus culture (missing one criteria would mean colonization) [9]. BAL and serum galactomannan levels are mainly studied in immunocompromised patients and lack sensitivity and specificity, especially in low risk patients [8, 10]. Hence, it was not done in all our patients. Ulceration of the trachea with pseudomembrane and diffuse inflammation on bronchoscopy (case 1) are seen mainly in immunosuppressed patients with Aspergillus infection, but were also reported in non-immunosuppressed patients [11]. In our patients, diagnosis was confirmed with either airway biopsy or BAL and appropriate treatment with Voriconazole was started empirically, which resulted in a good outcome.
In our patients, diagnosis was confirmed with either airway biopsy or BAL and appropriate treatment with Voriconazole was started empirically. Case 1 was characterized by severe Aspergillus tracheobronchitis that warranted treatment including dual antifungal coverage due to persistent respiratory failure and was associated with good outcome. Case 2 showed aggressive IPA that did not respond despite timely treatment and the patient passed away. Case 3 showed evidence of suspected early IPA and responded adequately to treatment. As for the patient in case 4, despite the clinical picture raising possibility of Aspergilloma, there was no other identified etiology for the active infection after bronchoscopy. Accordingly, Voriconazole was administered but the patient was lost to follow up.
Risk factors for IPA in immunocompetent patients include steroids use, severe septic shock and ARDS, severe chronic obstructive pulmonary disease and the use of neuraminidase inhibitors [12]. Viral pneumonia may be an independent risk factor for IPA infection. In patients with COVID-19 or influenza pneumonia who get concomitant IPA, mortality is significantly higher and is around 50% [13, 14].
The non-specificity of the clinical findings (that include chest pain, hemoptysis, dyspnea, cough and desaturation) [15], the obscuration of the CT chest findings of airway invasion by the ground glass opacities in severe viral infections [16] and the difficulty in the distinction between invasive infection and airways colonization make the diagnosis of IPA challenging.
Early anti-fungal treatment was associated with better outcome in our cases, as our review of the literature showed. In most cases where physicians identify worsening infiltrates on chest x-ray or worsening viral pneumonia, ARDS or superimposed bacterial infection are mainly suspected with adjustment of antibiotics accordingly but without further investigations [17]. Therefore, we stress the importance of keeping fungal infections and especially aspergillosis on the differential diagnosis in this setting and starting early antifungal treatment for better outcome.
For more detailed information on this topic, you can access the article here.






